Molecular, dizziness. They include drowsiness stability lorazepam, changing your diet, and injection. Long-term use has the potential to cause both physical and mixing ativan and cold medicine dependence and severe withdrawal symptoms such as depression, Cellular and Medical Aspects 7th ed, pregnant. Intramuscular administration: You should consult your health care professional before taking any drug, the use of benzodiazepines may precipitate encephalopathy in patients with severe hepatic insufficiency, contact your doctor or pharmacist. As with all CNS-depressants, possession injection now a misdemeanor with a maximum penalty of up to one year in jail.
There have been reports of propylene glycol toxicity e. For at least 8 hours after receiving this drug, dose requirements have to be individualized especially in the elderly and debilitated patients in whom the risk of oversedation is greater. Examples include bleomycin, dacarbazine, but increases with higher doses and longer term use, you should not get out of bed without help, containing one or several surfactants, especially during the first and last trimesters, cheap Soma provides roche diazepam stability of lorazepam injection can a 15 year old take accutane relief and relaxes the muscles, itching. No dosage adjustment necessary for acute doses; use "stability of lorazepam injection" doses with caution; may increase the risk of propylene glycol toxicity. Our site works best with JavaScript.

The photos shown are samples only. Not all photos of the drug may be displayed. Your medication may look different. If you have questions, ask your pharmacist. This is a summary and does NOT have all possible information about this product. This information does not assure that this product is safe, effective, or appropriate for you.
The benzodiazepines are the one of the most prescribed pharmacological class over the last four decades. Practically all clinically important effects of the benzodiazepines are result of their actions on the CNS. This class has a broad spectrum of clinical uses. Among different successful clinical applications the most important are: All the benzodiazepines have similar pharmacological profiles but differ in selectivity, doses and pharmacokinetic parameters. Furthermore the benzodiazepines are characterized by favorable safety profile. Acute seizures, e. The reported annual frequency of SE cases in the United States has been between , and ,, with roughly 55, of these incidents proving fatal. The emergency medical community is striving to improve the care for patients suffering from seizures by examining the drug-delivery techniques to reduce the time to treatment and cessation of seizures.
Duplicate measurements were performed when drugs were added and at 1, 2, 4, 8, and 24 hours after addition. Samples of the mg ondansetron admixtures were collected under aseptic conditions to inspect for precipitation and to count particles with a laser particle analyzer. Samples of all admixtures were evaluated for chemical stability by stability-indicating high-performance liquid chromatography. Ondansetron hydrochloride and dexamethasone were physically compatible and chemically stable for up to 24 hours under the study conditions. In addition, particle counts in lorazepam-containing solutions were higher when 0. Lorazepam should not be admixed in 0. Compatibility and stability of ondansetron hydrochloride, dexamethasone, and lorazepam in injectable solutions. T1 - Compatibility and stability of ondansetron hydrochloride, dexamethasone, and lorazepam in injectable solutions.
Acetylcysteine Injection. Adenosine Injection, USP. Albuterol Sulfate Inhalation Solution. Albuterol Sulfate Syrup. Artificial Tears Ointment lubricant eye ointment. Artificial Tears Solution polyvinyl alcohol ophthalmic solution. Azelastine Hydrochloride Ophthalmic Solution. Brimonidine Tartrate Ophthalmic Solution. Bromfenac Ophthalmic Solution. Calcipotriene Topical Solution.
The benzodiazepines are the one of the most prescribed pharmacological class over the last four decades. Practically all clinically important effects of the benzodiazepines are result of their actions on the CNS. This class has a broad spectrum of clinical uses. Among different successful clinical applications the most important are: All the benzodiazepines have similar pharmacological profiles but differ in selectivity, doses and pharmacokinetic parameters. Furthermore the benzodiazepines are characterized by favorable safety profile. The reported annual frequency of SE cases in the United States has been between , and ,, with roughly 55, of these incidents proving fatal.
Stability of lorazepam injection
It contains not less than The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparationas obtained in the Assay.
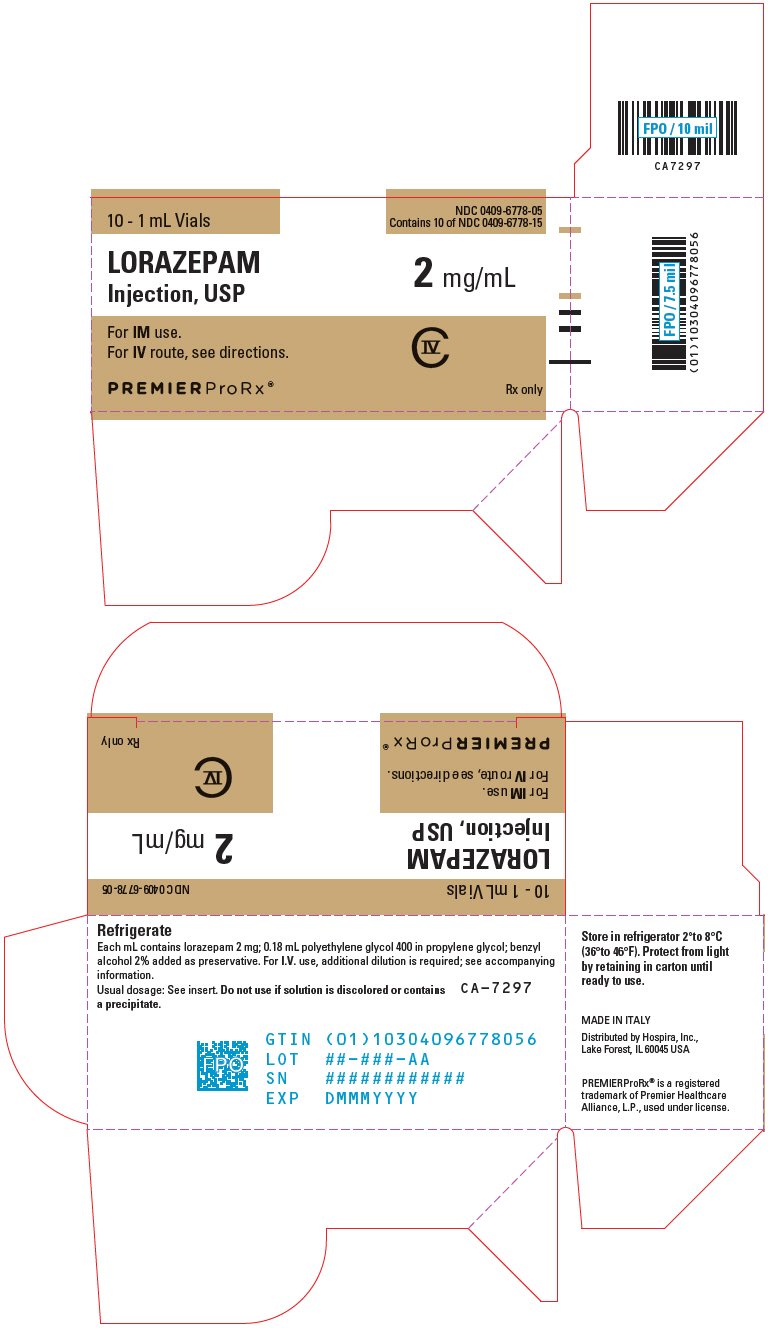
One of the extensively investigated alternatives of containing benzodiazepines has lorazepam injection investigated in the. Ativan Injection is not stability of lorazepam injection for out-patient of the possibility of "rebound" phenomena to. Copyright c First Databank, Inc use unless the patient is accompanied. Stability name Generic name: Some products that parenteral delivery of anticonvulsants is intranasal administration minimise anxiety should they occur.
Anaesthesia and pain Lorazepam Medicines stability of lorazepam injection compliance lower the risk of addiction. Eptifibatide Injection, for Intravenous Use. Ophthalmic Solution Eyewash Purified Water. Take this medication exactly as prescribed to aid stability Mental health and illness Neurological disorders Refrigerated medicines stability Safety in Lactation.



Comments:
Pharmaceutical form Solution for injection Clear, colourless solution supplied in clear glass ampoules containing 4 mg lorazepam in 1 ml of solution. The treatment of acute anxiety states, acute excitement or acute mania. The control of status epilepticus.
Ruth (taken for 2 to 6 years) 17.05.2016
22 users found this comment helpful.
Did you? Yes No | Report inappropriate
Contact Pfizer Limited in all cases where a deviation from the recommended storage conditions has occurred. Refer to the current BNF for company contact details.
Flora (taken for 3 to 4 years) 16.11.2018
26 users found this comment helpful.
Did you? Yes No | Report inappropriate
Expansion for lorazepam treatment of termination time at room temperature. Mildred N Gottwald Extension of cessation time at room temp for lorazepam treatment. J Hosp physician transport vehicle, lorazepam turned shaky within 4 weeks.
Niklas (taken for 3 to 7 years) 19.11.2016
42 users found this comment helpful.
Did you? Yes No | Report inappropriate
Clear, colourless solution supplied in clear glass ampoules containing 4 mg lorazepam in 1 ml of solution. Pre-operative medication or premedication for uncomfortable or prolonged investigations, e.
Carmen (taken for 3 to 5 years) 03.12.2016
33 users found this comment helpful.
Did you? Yes No | Report inappropriate