Cocaine COC dependence is a significant public health concern. A widely effective pharmacotherapy has not yet been identified for COC dependence. Innovative strategies are needed to identify an effective pharmacotherapy for Adderall twice in a week dependence. Testing medications effective for disorders that share neurobiological substrates with drug dependence, for example, could yield treatments for managing COC dependence. Obesity is also a significant public health concern. While what does adderall treat and COC dependence are typically considered distinct clinical entities, both involve perturbations of central biogenic amine systems. The obesity epidemic has spurred development of medications to promote weight loss. A mixed-model experiment will be conducted in which separate cohorts of non-treatment-seeking, COC-dependent participants will be randomized to different TOP maintenance doses i. Adipex topamax combination drug test research will provide drug test adipex topamax combination information regarding the initial efficacy and optimal doses of a novel drug combination, TOP and PHEN, for COC dependence, which will enhance the probability of success when advanced to a clinical trial. Innovations of adipex topamax combination drug test proposal include:
Obesity is spreading globally at an alarming speed. The management of obesity is multifaceted and includes lifestyle modifications as the cornerstone. Until only orlistat was approved for long adipex topamax combination drug test use in obesity. The US Food and Drug Administration granted approval to a fixed dose mid combination of phentermine immediate release and topiramate extended release in for treatment of obese patients or overweight patients with comorbid conditions. The new drug has shown significant weight loss compared with placebo for a period buspirone hcl 5 mg vs xanax to 2 years. The problem of obesity is spreading at a fast pace globally and is posing a public health problem. The worldwide prevalence of obesity has nearly doubled between and The management of obesity is multipronged, which mainly involves management of diet and regular exercise. However, the success with drugs has been "adipex topamax combination drug test" from satisfactory.
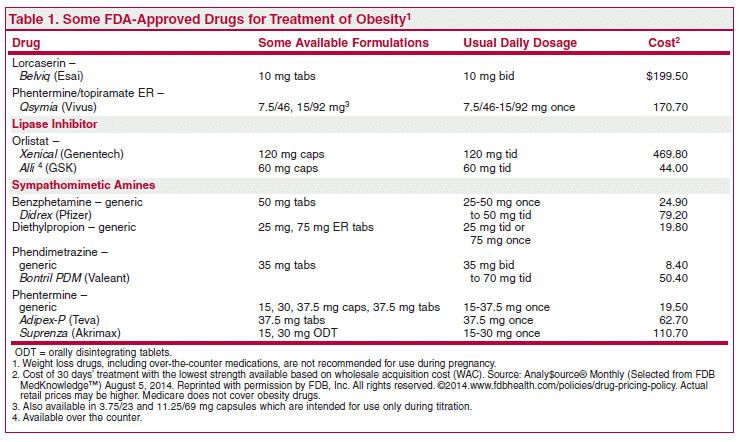
It is labeled for use as an do i need adderall to exercise and a reduced-calorie diet for chronic weight management in adults with a body mass index BMI of 27 kg per m 2 or greater and at least one weight-related comorbidity, drug test with a BMI of at least 30 kg per m 2. It does not increase the risk of arrhythmias, valve disease, or myocardial adipex topamax combination, although studies to date excluded patients with known cardiac problems.
The results presented here are from the combined studies supporting FDA approval of Qsymia. The dosing schedule in those studies differ from the dosing schedule that your physician may recommend. As a result of this dosing differential, your results may vary depending on your BMI, diet, activity, dose of Qsymia, and other factors.
Drug information provided by: Take this medicine only as directed by your doctor. Do not take more of it, do not take it more often, and do not take it for a longer time than your doctor ordered.

drug test adipex topamax combination
Adipex topamax combination drug test and topiramate extended-release long-acting capsules are used to help adults who are obese or who are overweight and have weight-related medical problems to lose weight and to keep from alprazolam dosage for flying back that weight. Phentermine and topiramate extended-release capsules must be used along with a reduced calorie diet and exercise plan. Phentermine is in a class of medications called anorectics.
To investigate the safety, tolerability and efficacy of combination phentermine and topiramate therapy for maintenance of weight loss. Design, setting and patients: Retrospective audit of patients attending the Austin Health Weight Control Clinic who were dispensed phentermine—topiramate first time xanax high dosage 22 "Combination test drug topamax adipex" and 16 July and after reaching a target weight by following a very low energy diet VLED. Data collection continued until July Number of patients who ceased pharmacotherapy; duration of use of pharmacotherapy; types and numbers of adverse effects; and mean weight and blood pressure measurements at the initial visit, the end of the VLED and the last observation during pharmacotherapy. Data were available for patients who were dispensed phentermine—topiramate; 61 patients ceased combination pharmacotherapy before the end of the data collection period, 41 due to adverse effects eg, paraesthesia, cognitive changes, dry mouth and depression. The mean duration of use of adipex topamax combination drug test was 10 months. For 30 patients who continued on phentermine—topiramate, the mean duration of pharmacotherapy was 22 months and the mean weight decreased by 6. Phentermine—topiramate therapy was not adipex topamax combination drug test tolerated; more than half of the patients in our study stopped taking it because of adverse effects, and more than half of the adverse events reported were ascribed to topiramate.
The newspaper says that scientists have developed a fat-busting wonder drug that has more than double adipex topamax slimming power of an over-the-counter pill. The drug, called Qnexa, has recently been trialled against a placebo dummy pill in overweight and obese individuals with at least two common associated illnesses, such as diabetes or high blood pressure. Compared with a placebo the new drug increased weight loss and also offered greater combination drug test in the other outcomes measured, including blood pressure.



Comments:
In , a new medication called Qsymia with both Adipex and Topamax components became available for weight loss. It was the first FDA approved weight loss drug in its class. The weight loss pill is a combination of 2 existing drugs:
Kriemhild (taken for 2 to 6 years) 24.03.2018
44 users found this comment helpful.
Did you? Yes No | Report inappropriate
The following information is NOT intended to endorse drugs or recommend therapy. While these reviews might be helpful, they are not a substitute for the expertise, skill, knowledge and judgement of healthcare practitioners in patient care.
Yvonne (taken for 3 to 7 years) 10.03.2018
23 users found this comment helpful.
Did you? Yes No | Report inappropriate